Specify oxidation coefficients.
SYNOPSIS
- oxide
-
orientation= ( 111 | 110 | 100 ) ( dry | wet )
[ lin.l.0 = n ] [ lin.l.e = n ] [ lin.h.0 = n ] [ lin.h.e = n ]
[ l.break = n ] [ l.pdep = n ]
[ par.l.0 = n ] [ par.l.e = n ] [ par.h.0 = n ] [ par.h.e = n ]
[ p.break = n ] [ p.pdep = n ]
[ ori.dep ] [ ori.fac = n ]
[ thinox.0 = n ] [ thinox.e = n ] [ thinox.l = n ]
[ hcl.pc = n ] [ hclT = string ] [ hclP = string ]
[ hcl.par = string ] [ hcl.lin = string ]
[ baf.dep ] [ baf.ebk = n ] [ baf.pe = n ] [ baf.ppe = n ]
[ baf.ne = n ] [ baf.nne = n ] [ baf.k0 = n ] [ baf.ke = n ]
[ stress.dep ] [ Vc = n ] [ Vr = n ] [ Vd = n ] [ Vt = n ]
[ Dlim = n ]
[ gamma = n ] [ alpha = n ]
[ henry.coeff = n | theta = n ]
[ ( silicon | oxide | nitride | poly | gaas | gas ) ]
[ ( /silicon | /oxide | /nitride | /poly | /gaas | /gas ) ]
[ diff.0 = n ] [ diff.e = n ] [ seg.0 = n ] [ seg.E = n ]
[ trn.0 = n ] [ trn.E = n ]
[ initial = n ] [ spread = n | mask.edge = n ]
[ erf.q = n ] [ erf.delta = n ] [ erf.lbb = n ] [ erf.h = n ] [ nit.thick = n ]
DESCRIPTION
All parameters relating to oxidation, and a few more besides, are specified here. Five oxide models are supported in this release: 1) an error-function fit to bird's beak shapes; 2) a parameterized error-function model from the literature; 3) a model where oxidant diffuses and the oxide boundaries move vertically at a rate determined by the local oxidant concentration; 4) a compressible viscous flow model, similar to 3 but in addition the new oxide formed at each time step forces the overlying oxide (and nitride) to flow; 5) an incompressible viscous flow model.
The error function is by far the fastest. It should be used for uniform oxidation, and is the most reliable for semi-recessed oxidation. It requires fitting data for the lateral spread of the bird's beak.
The parameterized model is by Guillemot [1]. The bird's beak length and nitride lifting were measured as a function of process conditions.
The diffusion model has no fitting parameters, but is only accurate when the growth is reasonably vertical - less than say 30 degree interface angle.
The compressible model is more accurate, but requires more computer time. It is still cheaper than the incompressible model, which uses many more grid points. The incompressible model is required whenever accurate stresses are desired.
Most coefficients need to know whether wet or dry oxidation is intended, and some need to know what the substrate orientation is.
- orientation
- The substrate orientation to which the coefficients specified apply. Required for orientation factor (see below) and thin oxide coefficients.
- dry | wet
- The type of oxidation to which the specified coefficients apply. Required for everything except for the one-dimensional coefficients and the volume ratio.
- lin.l.0, lin.l.e, lin.h.0, lin.h.e, l.break, l.pdep
- The linear rate coefficients (B/A). A doubly activated Arrhenius model is assumed. l.break is the temperature breakpoint between the lower and higher ranges, in degrees Celsius. lin.l.0 is the prefactor in microns/min, and lin.l.e is the activation energy in eV for the low temperature range. lin.h.0 and lin.h.e are the corresponding high temperature numbers. l.pdep is the exponent of the pressure dependence. The value given is taken to apply to <111> orientation and later adjusted by ori.fac according to the substrate orientation present.
- par.l.0, par.l.e, par.h.0, par.h.e, p.break, p.pdep
- The parabolic rate coefficients (B). Like the linear rate coefficients, but different.
- ori.dep, ori.fac
- The numerical coefficient ori.fac is the ratio of B/A on the specified orientation to that on the <111> orientation. The boolean parameter ori.dep (defaults true) specifies whether the local orientation at each point on the surface should be used to calculate B/A. If it is false, the substrate orientation is used at all points.
- thinox.0, thinox.e, thinox.l
- Coefficients for the thin oxide model proposed by Massoud [2]. thinox.0 is the prefactor in microns/min, thinox.e is the activation energy in eV, and thinox.l is the characteristic length in microns.
- hcl.pc, hclT, hclP, hcl.par, hcl.lin
- The numerical parameter hcl.pc is the percentage of HCl in the gas stream. It defaults to 0. The HCl dependence of the linear and parabolic coefficients is obtained from a look-up table specified in the model file.
The rows of the table are indexed by HCl percentage. The row entries can be specified with the parameter hclP, which is an array of numerical values, surrounded by double quotes and separated by spaces or commas. The columns are indexed by temperature. The column entries can be specified with the parameter hclT, which is an array of numerical values, surrounded by double quotes and separated by spaces or commas.
The dependence of B/A can be specified with the parameter hcl.lin, which is an array of numerical values, surrounded by double quotes and separated by spaces or commas. The number of entries in hcl.lin must be the product of the number of entries in hclP and hclT. The dependence of B can be specified with the parameter hcl.par, which is an array of numerical values, surrounded by double quotes and separated by spaces or commas. The number of entries in hcl.par must be the product of the number of entries in hclP and hclT.
- baf.dep, baf.ebk, baf.pe, baf.ppe, baf.ne, baf.nne, baf.k0, baf.ke
- These parameters relate to the doping dependence of the oxidation rate. The doping dependence is turned on if baf.dep is true. The linear rate coefficient, B/A, is assumed to depend on the Fermi level as follows [3].
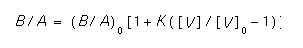
[V] is the total concentration of vacancies and K is an activated coefficient with prefactor baf.k0 and activation energy baf.ke. The remaining parameters above serve to compute [V]. The total concentration of vacancies [V] is the sum of the concentrations in each charge state (see vacancy).
- stress.dep, Vc, Vr, Vd, Vt, Dlim
- These parameters control the stress dependence of oxidation, which is only calculated under the viscous model. The parameter stress.dep turns on the dependence. The parameter Vc is the activation volume of viscosity. The parameter Vr is the activation volume of the reaction rate with respect to normal stress. The parameter Vt is the activation volume of the reaction rate with respect to tangential stress. The parameter Vd is the activation volume of oxidant diffusion with respect to pressure. The parameter Dlim is the maximum increase of diffusion permitted under tensile stress.
- gamma
- The parameter gamma is the oxidant surface energy in ergs/cm2, which controls reflow.
- alpha
- The volume expansion ratio between material 1 and material 2. In a nutshell, alpha oxide /silicon is 2.2, and alpha x /y is 1.0 for everything else (until someone tells us otherwise).
- silicon | oxide | nitride | poly | gaas | gas
- What material 1 is.
- /silicon | /oxide | /nitride | /poly | /gaas | /gas
- What material 2 is.
- henry.coeff | theta
- Henry's coefficient is the solubility of oxidant in material 1 at one atmosphere, per cubic centimeter. Theta is the number of oxygen atoms incorporated in a cubic centimeter of oxide. Note: Don't change these unless you really know what you are doing. Change the Deal-Grove coefficients instead.
- diff.0, diff.e, seg.0, seg.E, trn.0, trn.E
- The diffusion coefficients of oxidant in material 1, and the boundary coefficients ("transport" and "segregation") from material 1 to material 2. See the note above. For the record, diff.0 is the diffusivity prefactor in cm2/sec, diff.e is the energy in eV. The transport coefficient represents the gas phase mass-transfer coefficient in terms of concentrations in the solid at the oxide-gas interface, the chemical surface-reaction rate constant at the oxide-silicon surface, and a regular diffusive transport coefficient at other interfaces. The segregation coefficient is 1 at the oxide-gas interface, infinity at the oxide-silicon interface, and represents a regular segregation coefficient at other interfaces.
- initial
- The thickness of the existing oxide at the start of oxidation, default 2 nanommeters. If the wafer is bare, an oxide layer of this thickness is deposited before oxidation begins.
- spread | mask.edge
- These coefficients are only used in the error-function approximation to a bird's beak shape. Spread is the relative lateral to vertical extension, defaults to 1. It's a fitting parameter to make erfc birds look realistic. Mask.edge is the position of the mask edge in microns, and defaults to negative infinity. Oxide grows to the right of the mask edge.
- erf.q, erf.delta, erf.lbb, erf.h, nit.thick
- This group of parameters all apply to the "erfg" model. See Guillemot et al. [1] for their interpretation.
- erf.q, erf.delta
- The delta and q parameters for the "erfg" model. These probably do not need to be changed, but they're available if you want them.
- erf.lbb
- The erf.lbb parameter is the length of the bird's beak. It can be specified as an expression in Eox (the field oxide thickness (microns)), eox (the pad oxide thickness (microns)), Tox (the oxidation temperature (Kelvin)), and en (the nitride thickness (microns)). The published expression can be found in the models file. Specifying "erf.llbb=Eox" for instance would give a lateral spread equal to the field thickness, similar to the Hee-Gook Lee model with a spread of 1.
- erf.h
- The erf.h parameter is the ratio of the nitride lifting to the field oxide thickness. (It corresponds to the Guillemot "H" parameter [1] except that it is normalized to the field oxide thickness). Again it is specified as an expression of Eox, eox, Tox, en.
- nit.thick
- The nitride thickness to substitute for the parameter "en".
EXAMPLES
Look at the models file for a huge example.
BUGS
In increasing order of severity:
- If a required parameter is omitted, like orientation when a linear rate coefficient is being specified, the statement is ignored without warning.
- The volume expansion data should be on the material statement.
- Oxidant in materials other than oxide is allowed to diffuse and segregate, but its concentration is then ignored (no oxynitridation, for instance). The diffusion and transport coefficients in oxide to gas and silicon are derived from the Deal-Grove coefficients, so these parameters are ignored if read from input statements.
- The non-analytic oxide models don't account for the thin oxide correction in dry oxygen.
- The analytic models use the "thickness" of the oxide to compute the growth rate. In addition, the "erfg" model uses the nitride thickness. These values are NOT inferred from the structure. Instead, the nitride thickness is taken from the user on the oxide command, and the oxide thickness is computing by adding the total oxide grown to the initial oxide thickness specified on the oxide command. If the structure at the start of diffusion has more than the default 20 angstroms of native oxide on it, that thickness must be specified by the user. Beware of this when continuing a diffusion by any means (e.g. after reading in a previous structure). A "diffuse" followed by "diffuse continue=t" will however do the right thing.
REFERENCES
- N. Guillemot, G. Pananakakis and P. Chenevier, "A New Analytical Model of the ``Bird's Beak''," IEEE Transactions on Electron Devices, ED-34, 1987.
- H. Z. Massoud, J. D. Plummer and E.A. Irene, "Thermal Oxidation of Silicon in Dry Oxygen Growth Rate Enhancement in the Thin Regime," J. Electrochem. Soc., 132, p. 2685, 1985.
- C. P. Ho and J. D. Plummer, "Si/SiO2 Interface Oxidation Kinetics: A Physical Model for the Influence of High Substrate Doping Levels, Parts I and II," J. Electrochem. Soc., 126, p. 1523, 1979.