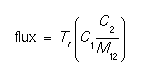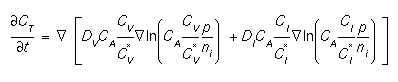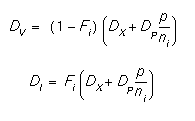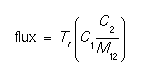Set the coefficients of boron kinetics.
SYNOPSIS
- boron
-
( silicon | oxide | oxynit | nitride | gas | poly | gaas )
[ Dix.0 = n ] [ Dix.E = n ]
[ Dip.0 = n ] [ Dip.E = n ]
[ Fi = n ]
[ implanted ] [ grown.in ]
[ ss.clear ] [ ss.temp = n ] [ ss.conc = n ]
[ ( /silicon | /oxide | /oxynitr | /nitride | /gas | /poly | /gaas ) ]
[ Seg.0 = n ] [ Seg.E = n ]
[ Trn.0 = n ] [ Trn.E = n ]
[ ( donor | acceptor ) ]
DESCRIPTION
This statement allows the user to specify values for coefficients of boron diffusion and segregation. The diffusion equation for boron is [1,2]:
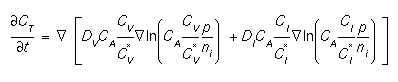
where CT and CA are the total chemical and active concentrations of boron, CA is the total electrically active concentration of boron, CV and CI are the vacancy and interstitial concentrations, the superscript * refers to the equilibrium value, DV and DI are the diffusivities with vacancies and interstitials, and p and ni refer to the hole concentration and the intrinsic electron concentration respectively. The diffusivities are given by:
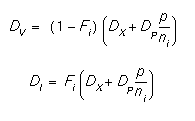
DX and DP are described in greater detail below.
The segregation at material interfaces is computed using the following expression:
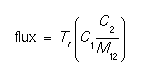
where C1 and C2 are the concentrations in material 1 and 2 respectively, and the M12 and Tr terms are computed using expressions shown below with the parameters of the models.
- silicon, oxide, oxynitr, nitride, gas, poly, gaas
- These allow the specification of parameters for that material. Only one of these can be specified per statement. The parameters specified in that statement will apply in the material listed. These parameters specify which material is material 1 for the segregation terms.
- Dix.0, Dix.E
- These floating point parameters allow the specification of DX, the boron diffusivity with neutral defects. Dix.0 is the pre-exponential constant and Dix.E is the activation energy. Dix.0 defaults to 0.28 cm2/sec in silicon, and Dix.E defaults to 3.46 eV in silicon [3]. DX is calculated using a standard Arrhenius relationship.
- Dip.0, Dip.E
- These floating point parameters allow the specification of DP, the boron diffusivity with singly positive defects. Dip.0 is the pre-exponential constant and Dip.E is the activation energy. Dip.0 defaults to 0.23 cm2/sec in silicon, and Dip.E defaults to 3.46 eV in silicon [3]. DP is calculated using a standard Arrhenius relationship.
- Fi
- This parameter allows the specification of the fractional interstitialcy. This value indicates whether boron diffuses through interaction with interstitials or vacancies. The value of Fi defaults to 0.8 [4].
- implanted, grown.in
- Specifies whether the Dix, Dip, or Fi coefficients apply to implanted or grown-in boron. If neither is specified then a specified parameter applies to both.
- ss.clear
- This parameter clears the currently stored solid solubility data.
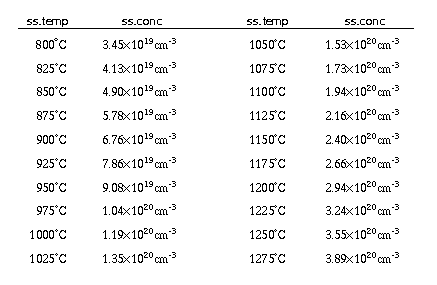
- ss.temp, ss.conc
- These parameters add a single temperature solid solubility concentration point to those already stored. The default values are [5].
- /silicon, /oxide, /oxynit, /nitride, /gas, /poly, /gaas
- These parameters specify material 2. Only one of the these parameters can be specified at one time.
- Seg.0, Seg.E
- These parameters allow the computation of the equilibrium segregation concentrations. The segregation constant follows an Arrhenius relationship.
- Trn.0, Trn.E
- These parameters allow the specification of the transport velocity across the interface given. The units are in cm/sec. The transfer coefficient follows an Arrhenius relationship.
- donor, acceptor
- These parameters determine whether the impurity is to be treated as a donor or as an acceptor in a semiconductor material. These parameters are not presently material specific. By default boron is an acceptor.
EXAMPLES
- boron silicon Dix.0=0.28 Dix.E=3.46
- This command changes the neutral defect diffusivity in silicon.
- boron silicon /oxide Seg.0=1126.0 Seg.E=0.91 Trn.0=1.66e-7
- This command will change the segregation parameters at the silicon - silicon dioxide interface. The silicon concentration will be half the oxide concentration in equilibrium at 1100C.
BUGS
As far as the implemented models are physically correct, there are no known bugs.
REFERENCES
- M. E. Law and J. R. Pfiester, "Low Temperature Annealing of Arsenic/Phosphorus Junctions," IEEE Trans. on Elec. Dev., 38(2), p. 278, 1991.
- D. Mathiot and J. C. Pfister, "Dopant Diffusion in Silicon: A consistent view involving nonequilibrium defects," J. Appl. Phys., 55(10), p. 3518, 1984.
- G. P. Barbuscia, G. Chin, R. W. Dutton, T. Alvarez and L. Arledge, "Modeling of Polysilicon Dopant Diffusion for Shallow Junction Bipolar Technology," International Electron Devices Meeting, San Francisco, p. 757, 1984.
- P.A. Packan and J.D. Plummer, "Temperature and Time Dependence of B and P Diffusion in Si During Surface Oxidation," J. Appl. Phys., 68(8), 1990.
- A. Armigliato, D. Nobili, P. Ostoja, M. Servidori and S. Solmi, "Solubility and Precipitation of Boron in Silicon and Supersaturation Resulting by Thermal Predeposition," J. Appl. Phys.
SEE ALSO
The
antimony,
arsenic,
interstitial,
phosphorus,
ane vacancy statements.